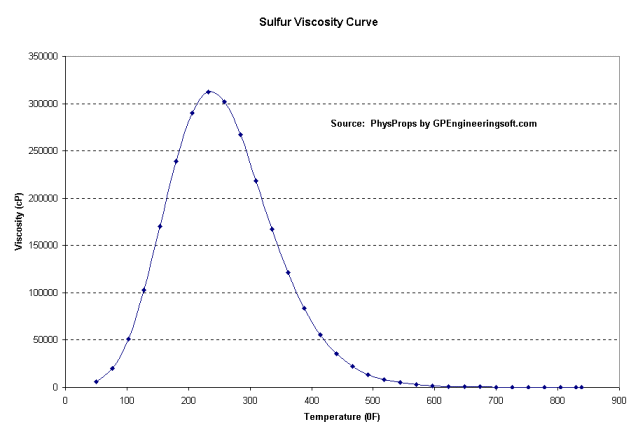
Sulfur, oil and gas by-product information webpage
Sulfur, oil and gas by-product information webpage
In this discussion:
http://peakoil.com/fortopic1917.html
"Saudi extra barrels wrong kind of crude!"
big_rc asked:
"what do the refineries do with the sulphur they remove from the oil?"
so I made this web page:
http://www.cuug.ab.ca/kmcclary/sulfur/
http://peakoil.com/fortopic1917.html
"Saudi extra barrels wrong kind of crude!"
big_rc asked:
"what do the refineries do with the sulphur they remove from the oil?"
so I made this web page:
http://www.cuug.ab.ca/kmcclary/sulfur/
-

Keith_McClary - Light Sweet Crude
- Posts: 7344
- Joined: Wed 21 Jul 2004, 03:00:00
- Location: Suburban tar sands
Nice work Keith!
More from here.
$this->bbcode_second_pass_quote('', 'S')ulfuric acid is prepared industrially by the reaction of water with sulfur trioxide, which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. The lead chamber process is used to produce much of the acid used to make fertilizers. It produces a relatively dilute acid (62% - 78%). The contact process produces a more concentrated acid but requires purer raw materials and the use of expensive catalysts. Some sulfuric acid is also made from ferrous sulfate waste solutions from pickling iron and steel and from waste acid sludge from oil refineries.
The uses of sulfuric acid are so varied that the volume of its production provides an approximate index of general industrial activity. Its main use is in phosphate fertilizer production, both superphosphate of lime and ammonium sulfate. It is widely also used to manufacture chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, drugs, other acids, parchment paper, glue and wood preservatives. It is used in the purification of petroleum to wash impurities out of gasoline and other refinery products.Sulfuric acid is used in processing metals, e.g., in pickling (cleaning) of metal, electroplating baths, nonferrous metallurgy. Rayon is made with sulfuric acid. In one of its most familiar applications, it serves as the electrolyte in the lead-acid storage battery commonly used in motor vehicles (acid for this use, containing about 33% H2SO4 and with specific gravity about 1.25, is often called battery acid).
More from here.
$this->bbcode_second_pass_quote('', 'S')ulfuric acid is prepared industrially by the reaction of water with sulfur trioxide, which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. The lead chamber process is used to produce much of the acid used to make fertilizers. It produces a relatively dilute acid (62% - 78%). The contact process produces a more concentrated acid but requires purer raw materials and the use of expensive catalysts. Some sulfuric acid is also made from ferrous sulfate waste solutions from pickling iron and steel and from waste acid sludge from oil refineries.
The uses of sulfuric acid are so varied that the volume of its production provides an approximate index of general industrial activity. Its main use is in phosphate fertilizer production, both superphosphate of lime and ammonium sulfate. It is widely also used to manufacture chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, drugs, other acids, parchment paper, glue and wood preservatives. It is used in the purification of petroleum to wash impurities out of gasoline and other refinery products.Sulfuric acid is used in processing metals, e.g., in pickling (cleaning) of metal, electroplating baths, nonferrous metallurgy. Rayon is made with sulfuric acid. In one of its most familiar applications, it serves as the electrolyte in the lead-acid storage battery commonly used in motor vehicles (acid for this use, containing about 33% H2SO4 and with specific gravity about 1.25, is often called battery acid).
And here.
$this->bbcode_second_pass_quote('', 'T')he largest sources of elemental sulfur are petroleum refining and natural gas processing at numerous facilities throughout the United States. Elemental sulfur is mined at a few locations worldwide. Smaller quantities of sulfur are recovered as sulfuric acid at nonferrous metal smelters, and minor amounts are recovered at coking operations. Between 11 and 12 Mt/yr of domestic sulfur in all forms are produced. The United States imports about 3 Mt/yr of sulfur as elemental sulfur and sulfuric acid. Exports total less than 1 Mt/yr. The majority of U.S. imports come from Canada, the largest sulfur exporter in the world. Annual apparent consumption is almost 14 Mt.
Last edited by rowante on Wed 06 Oct 2004, 08:46:23, edited 1 time in total.
- rowante
- Lignite

- Posts: 244
- Joined: Tue 06 Apr 2004, 03:00:00
- Location: Sydney, Australia
This website never ceases to amaze me. I learn something new almost everytime I log on here.
Thanks for the info !!
Thanks for the info !!
Simon's Law: Everything put together falls apart sooner or later.
I don't think of all the misery, but of all the beauty that still remains.--Anne Frank
I don't think of all the misery, but of all the beauty that still remains.--Anne Frank
-

big_rc - Coal

- Posts: 478
- Joined: Sat 17 Jul 2004, 03:00:00
- Location: Amerika (most of the time)
Re: sulfur, oil and gas by-product
$this->bbcode_second_pass_quote('Keith_McClary', 'I')n this discussion:
http://www.cuug.ab.ca/kmcclary/sulfur/
http://www.cuug.ab.ca/kmcclary/sulfur/
great webpage!
Very informative clear and it gives you the idea within half a minute
my compliments
-

Rembrandt - Peat

- Posts: 58
- Joined: Sat 21 Aug 2004, 03:00:00
Re: sulfur, oil and gas by-product
Russian Scientist Suggests Burning Sulfur in Stratosphere to Fight Global Warming
Just to put this in perspective, the amount of sulfur he is proposing to burn is about half of this stockpile:
http://www.cuug.ab.ca/kmcclary/sulfur/
BTW, the stockpile has been almost all melted down and shipped away in the year since I made the webpage.
Just to put this in perspective, the amount of sulfur he is proposing to burn is about half of this stockpile:
http://www.cuug.ab.ca/kmcclary/sulfur/
BTW, the stockpile has been almost all melted down and shipped away in the year since I made the webpage.
-

Keith_McClary - Light Sweet Crude
- Posts: 7344
- Joined: Wed 21 Jul 2004, 03:00:00
- Location: Suburban tar sands
Re: Sulfur, oil and gas by-product information webpage
Lets see, am I hearing this right?
We removed all the sulfer from our oil to save the air,
Now we have to burn it in the air to save the planet?
Who screwed this one up?
But by Removing all the sulfer from the oil processing refineries to SAVE the ecology, we have then caused the global warming?
Reminds me of ants I saw when I was little, a couple thousand were stacking up sand in one area and another couple thousand were unstacking it. the unstackers were winning.
But reality is probably the truth:
Russia probably has high sulfer oil, and it is expensive to extract it, so the Russians paid a scientist to come up with a reason for leaving sulfer in the oil and this is their explanation, thus raising Russias high sulfur oil value with the stroke of a pen.
Hey I could become a politician.
Except I would have to overcome my problem with honesty.
We removed all the sulfer from our oil to save the air,
Now we have to burn it in the air to save the planet?
Who screwed this one up?
But by Removing all the sulfer from the oil processing refineries to SAVE the ecology, we have then caused the global warming?
Reminds me of ants I saw when I was little, a couple thousand were stacking up sand in one area and another couple thousand were unstacking it. the unstackers were winning.
But reality is probably the truth:
Russia probably has high sulfer oil, and it is expensive to extract it, so the Russians paid a scientist to come up with a reason for leaving sulfer in the oil and this is their explanation, thus raising Russias high sulfur oil value with the stroke of a pen.
Hey I could become a politician.
Except I would have to overcome my problem with honesty.
-

grabby - Heavy Crude

- Posts: 1291
- Joined: Tue 08 Nov 2005, 04:00:00
Re: Sulfur, oil and gas by-product information webpage
Sulfur in other fuels causes acid rain. I guess it's only sulphur in the stratosphere that is "safe". Of course, it may not be so safe after all. In the end, it all depends on whether the remedy is worse or better than the disease.
-

Doly - Expert

- Posts: 4370
- Joined: Fri 03 Dec 2004, 04:00:00
Re: Sulfur, oil and gas by-product information webpage
Much of our natural gas is loaded with hydrogen sulfide. Evil, deadly gas, second only to HCN in toxicity.
The old way was to use a process to remove it from the gas using an amine process similar to that used to remove CO2 from gas. THe H2S was then burned in a flare or candlestick incinerator. (H2S is very flamable) this resulted in a lot of SO2 emissions. (not to mention the H2S that was not burned.
More modern methods used something called a Sulfur Recovery Unit (SRU) Sulfur had economic value for use for fertilizer and such. In the Klaus process, 2/3 of the H2S was burned in the air to make SO2, then reacted with the other 1/3 of the H2S on a catalyst to make elemental sulfur and water. Pretty slick. The tail gas from this process was run through the flare or candlestick as above.
Since Sulfur is bad for our planet, efforts have been underway to reduce the amount released into the air. The above two process have been limited by both environmental regulations and a glut in the global sulfur market. It's hard to get rid of sulfur nowadays.
So, the more modern method is to strip the H2S out of the gas, then reinject it into the ground. The H2S is 40% or more, balance CO2, then run down a wellbore, and gone.
One of the more interesting places I've been liquifies the H2S prior to reinjection. The same place scavenges the waste gas from the processing of NG and runs a pretty big power plant of the waste gas.
On another note, most of the CO2 stripped from gas (and it is a LOT of CO2) is blown into the sky as a waste product. The above mentioned plant shipped their waste CO2 hundreds of miles (pipeline) to reinject into oil-bearing formations to improve production. I'm not going to name them by name, but they don't (publicly) believe in PO. Although they are a very large and very evil corporation, they eat every part of the buffalo.
The old way was to use a process to remove it from the gas using an amine process similar to that used to remove CO2 from gas. THe H2S was then burned in a flare or candlestick incinerator. (H2S is very flamable) this resulted in a lot of SO2 emissions. (not to mention the H2S that was not burned.
More modern methods used something called a Sulfur Recovery Unit (SRU) Sulfur had economic value for use for fertilizer and such. In the Klaus process, 2/3 of the H2S was burned in the air to make SO2, then reacted with the other 1/3 of the H2S on a catalyst to make elemental sulfur and water. Pretty slick. The tail gas from this process was run through the flare or candlestick as above.
Since Sulfur is bad for our planet, efforts have been underway to reduce the amount released into the air. The above two process have been limited by both environmental regulations and a glut in the global sulfur market. It's hard to get rid of sulfur nowadays.
So, the more modern method is to strip the H2S out of the gas, then reinject it into the ground. The H2S is 40% or more, balance CO2, then run down a wellbore, and gone.
One of the more interesting places I've been liquifies the H2S prior to reinjection. The same place scavenges the waste gas from the processing of NG and runs a pretty big power plant of the waste gas.
On another note, most of the CO2 stripped from gas (and it is a LOT of CO2) is blown into the sky as a waste product. The above mentioned plant shipped their waste CO2 hundreds of miles (pipeline) to reinject into oil-bearing formations to improve production. I'm not going to name them by name, but they don't (publicly) believe in PO. Although they are a very large and very evil corporation, they eat every part of the buffalo.
Last edited by aflatoxin on Fri 17 Mar 2006, 01:50:27, edited 1 time in total.
-

aflatoxin - Lignite

- Posts: 278
- Joined: Sun 31 Jul 2005, 03:00:00
Re: Sulfur, oil and gas by-product information webpage
Another place I worked at took H2S from a nearby NG processing plant, then burned it to make SO2>SO3>(H2SO4) (Anhydrous sulfuric acid).
Pretty scary stuff. They took this and put it into a reactor with common salt (NaCl) to make HCl gas> hydrochloric acid. This was used to pour down oil wells to dissolve limestone and improve production.
This was, and is, hands down, the most dangerous place I've ever been.
I only got gassed by the HCl when the line feeding the RV to the tails scrubber blew, but I barely made it out before I passed out. The H2SO4 contaminates in the gas cloud dissolved my tee-shirt. Were it not for my air-pac, I probably would have died. Rumor has it that people came off the tower only wearing their zipper.
(BTW, this plant was in the US, it was closed in 1999 when low oil prices killed the demand for HCl)
Pretty scary stuff. They took this and put it into a reactor with common salt (NaCl) to make HCl gas> hydrochloric acid. This was used to pour down oil wells to dissolve limestone and improve production.
This was, and is, hands down, the most dangerous place I've ever been.
I only got gassed by the HCl when the line feeding the RV to the tails scrubber blew, but I barely made it out before I passed out. The H2SO4 contaminates in the gas cloud dissolved my tee-shirt. Were it not for my air-pac, I probably would have died. Rumor has it that people came off the tower only wearing their zipper.
(BTW, this plant was in the US, it was closed in 1999 when low oil prices killed the demand for HCl)
-

aflatoxin - Lignite

- Posts: 278
- Joined: Sun 31 Jul 2005, 03:00:00
Re: Sulfur, oil and gas by-product information webpage
$this->bbcode_second_pass_quote('aflatoxin', ' ')Although they are a very large and very evil corporation, they eat every part of the buffalo.
You mean Kerr-Mc Evil?-

Keith_McClary - Light Sweet Crude
- Posts: 7344
- Joined: Wed 21 Jul 2004, 03:00:00
- Location: Suburban tar sands